Abstract
Introduction: Immunoglobulin light chain (AL) amyloidosis is associated with significant morbidity and mortality. Several models were developed to allow survival prediction, but head-to-head model comparison is lacking.
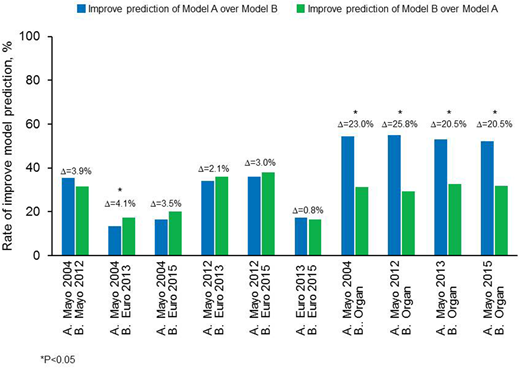
Patients and Methods: Five prognostic models were compared: Mayo 2004; Mayo 2012; 2013 European modification of Mayo 2004 (where Mayo 2004 stage 3 is subdivided into 3 subgroups based on NT-proBNP at 8500 ng/L and systolic blood pressure at 100 mmHg); 2015 European modification of Mayo 2004 (where Mayo 2004 stage 3 is subdivided into 2 subgroups based on NT-proBNP at 8500 ng/L); and organ model (a model based on number of involved organs). All patients with available baseline data for the 5 models were included in this study (n=1005). Improvements in predictive accuracy of the various models were determined by calculating a net improvement in survival prediction for each pair of prognostic models. Net improvement was considered as the proportion where model A outperforms model B minus the proportion where model B outperforms model A.
Results: For all models, there was increasing risk for death with increasing stage. The organ model was the least powerful system with a hazard ratio of 2.8 for ≥4 organs compared to 1 involved organ. The organ model was consistently outperformed by the other 4 systems. In the cohort as a whole, the biomarker models (Mayo 2004; Mayo 2012; European 2013; European 2015) were comparable in ability to predict survival (Figure). However, a slightly superior performance of the European 2013 modification of the Mayo 2004 system was seen over the Mayo 2004 system in the group as a whole (net improvement 4.1%, P=0.023) and when restricted to non-ASCT population (net improvement 6.3% P=0.014). The European 2015 modification performed slightly better than the Mayo 2004 system in the ASCT population (net improvement 3.4%, P=0.025). 1-year mortality was better predicted by the European models (net improvement 11-14%, P<0.05), while the Mayo 2012 had better survival prediction to all other biomarker models in 3-year landmark comparison (net improvement 7-9%, P<0.05).
Conclusion: Using biomarker based staging is a clear improvement over the organ staging system. The different biomarker staging systems' abilities to predict for survival were relatively comparable, solidifying the importance of soluble cardiac biomarkers and free light chain measurement as prognosticators in AL amyloidosis. Mayo 2004-based models better predict for early death while Mayo 2012 model has a better prediction for long-term survival.
Gertz:Ionis: Honoraria; celgene: Consultancy; spectrum: Consultancy, Honoraria; janssen: Consultancy; Amgen: Consultancy; Abbvie: Consultancy; Medscape: Consultancy; annexon: Consultancy; Teva: Consultancy; Research to Practice: Consultancy; Apellis: Consultancy; Alnylam: Honoraria; Prothena: Honoraria; Physicians Education Resource: Consultancy. Lacy:Celgene: Research Funding. Dingli:Millennium Takeda: Research Funding; Millennium Takeda: Research Funding; Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.; Alexion Pharmaceuticals, Inc.: Other: Participates in the International PNH Registry (for Mayo Clinic, Rochester) for Alexion Pharmaceuticals, Inc.. Kapoor:Celgene: Research Funding; Takeda: Research Funding. Russell:Vyriad: Equity Ownership. Kumar:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Dispenzieri:Celgene, Takeda, Prothena, Jannsen, Pfizer, Alnylam, GSK: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal